Buffer solution—
Dissolve 1.95 g of 2-(
N-morpholino)-ethanesulfonic acid and 1.22 g of tris(hydroxymethyl)aminomethane in 170 mL of water. Adjust with 6 N sodium hydroxide to a pH of 8.2, dilute with water to 200 mL, and mix.
[NOTE—It is important to adjust the pH to 8.2 at 24

. If the
Buffer solution temperature is 20

, adjust the pH to 8.3; if the temperature is 28

, adjust the pH to 8.1. For deviations between 20

and 28

, adjust by interpolation.
]
Enzyme purity—
To ensure the absence of undesirable enzymatic activities and the presence of desirable enzymatic activities, proceed as directed for
Test preparations and
Procedure using the substrates listed in the following table in place of Psyllium Hemicellulose.
| Substrate |
Weight in g |
Activity Tested |
| Pectin |
0.2 |
Pectinase |
| Arabinogalactan |
0.2 |
Hemicellulase |
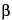 -Glucan -Glucan |
0.2 |
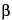 -Glucanase -Glucanase |
| Wheat starch |
1.0 |
 -Amylase and -Amylase and
amyloglucosidase |
| Corn starch |
1.0 |
 -Amylase and -Amylase and
amyloglucosidase |
| Casein |
0.3 |
Protease |
The enzyme preparation is suitable if more than 90% of the original weight of pectin, arabinogalactan, and
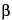
-glucan is recovered; not more than 2% of the original weight of casein and corn starch is recovered; and not more than 1% of the original weight of wheat starch is recovered.
[NOTE—Test the enzyme purity of every new lot of enzyme and at 6-month intervals thereafter.
]
Procedure—
Treat each preparation in the following manner. Add 40 mL of
Buffer solution to the beaker.
[NOTE—For the
Test preparation, stir until Psyllium Hemicellulose is completely dispersed.
] Add 125 µL of heat-stable

-amylase solution, and stir to ensure uniform mixing. Cover the beaker with aluminum foil, and incubate over a water bath maintained at 95

to 100

for 15 minutes, with continuous agitation.
[NOTE—Start timing once the water bath temperature reaches 95

; a total time of 35 minutes is usually sufficient.
] Remove the beaker from the water bath, and cool to 60

. Remove the aluminum foil, scrape any ring from inside the beaker, and disperse any gels in the bottom of the beaker with a spatula. Rinse the walls of the beaker and the spatula with 10 mL of water, collecting the rinsings in the beaker. Add 500 µL of
Protease solution. Cover with aluminum foil, and incubate over a water bath maintained at 60 ± 3

for 30 minutes with continuous agitation.
[NOTE—Start timing when the bath temperature reaches 60

.
] Remove the foil, and transfer 5 mL of
Acid solution while stirring. Adjust, if necessary, with 1 N sodium hydroxide or 1 N hydrochloric acid to a pH of 4.28 ± 0.07 at 60

.
[NOTE—It is important to adjust the pH to 4.28 while the solution in the beaker is maintained at 60

, otherwise the pH will increase at lower temperatures.
] Add 150 µL of amyloglucosidase solution while stirring. Cover with aluminum foil, and incubate over a water bath maintained at 60 ± 3

for 30 minutes with constant agitation.
[NOTE—Start timing once the water bath reaches 60

.
] Transfer approximately 40 mL of the beaker contents to a 50-mL centrifuge tube, and sonicate the tube contents for 3 minutes.
* Centrifuge at 10,000–14,000 rpm for 10 minutes. Carefully pour the supernatant into an appropriately labeled 600-mL tared beaker. Do not disturb any pellet in the bottom of the centrifuge tube. Add the remaining sample from the original 400-mL beaker into the centrifuge tube still containing the pellet. Rinse the 400-mL beaker with 15–20 mL of water, and add the rinsing to the 50-mL centrifuge tube. Centrifuge the sample at 10,000–14,000 rpm for 10 minutes. Carefully pour the supernatant into the 600-mL beaker containing the first supernatant. Add 390 mL (measured before heating) of alcohol at 60

to the 600-mL beaker. Cover the beaker, and allow to stand for at least 1 hour to form a precipitate.
Place 3 g of chromatographic siliceous earth into a clean air-dried crucible with a fritted disk. Heat the crucible containing chromatographic siliceous earth at 525º in a muffle furnace for at least 4 hours. Cool. Pass deionized water through the crucible while applying constant suction. Rinse with acetone, and allow to air-dry. Store the crucible in a convection oven at approximately 130º for at least 2 hours before use. Weigh the prepared crucible to 0.1 mg before use. Wet the chromatographic siliceous earth in the crucible using a stream of
Alcohol solution from a washing bottle, and apply suction to evenly distribute the chromatographic siliceous earth over the fritted disk. Maintaining the suction, transfer the supernatant and precipitate from the beaker to the crucible, and filter. Transfer any solid residue in the beaker with the aid of
Alcohol solution. [NOTE—In some cases, gums may form during filtration, trapping liquid in the residue. If so, break the surface film with a spatula to improve filtration.
] Wash the residue in the crucible sequentially with 30 mL of
Alcohol solution, 20 mL of alcohol, and 20 mL of acetone. Dry the crucible containing the residue at 100

in a convection oven for at least 4 hours, cool to room temperature in a desiccator.
Determine the weight of the residue (R).
Use one of the duplicate residues from the
Test preparations and one of the blank residues from the
Blank preparations to determine the protein content, in mg, by placing the residue in a 500-mL Kjeldahl flask, and proceeding as directed for
Method I under
Nitrogen Determination  461
461
. The protein content is determined by multiplying the content of nitrogen found by 6.25. Incinerate the residue from the remaining duplicate of the
Test preparation and the
Blank preparation as directed for
Total Ash under
Articles of Botanical Origin  561
561
at a reduced temperature of 525

, and determine the ash content as directed. Calculate the corrected average weight of the blank, in mg,
B, by the formula:
RB – P B – AB,
in which RB is the weight, in mg, of the average blank residue for duplicate blank determinations; PB is the content, in mg, of protein found in the blank; and AB is the content, in mg, of ash found in the blank. Calculate the content of soluble dietary fiber, in percentage, by the formula:
100(RU – PU – AU – B)/WU,
in which RU is the weight, in mg, of average residue for the duplicate Test preparations; PU is the content of protein, in mg, found in the Psyllium Hemicellulose; AU is the content of ash, in mg, found in the Psyllium Hemicellulose; B is the average weight of the blank as calculated above; and WU is the average weight, in mg, of the Psyllium Hemicellulose taken.