Packaging and storage—
Preserve in tight containers, protected from light. Store at temperatures not exceeding 25

.
Labeling—
Where it is intended for use in parenteral nutrition, it is so labeled.
Appearance—
The substance is clear and not more intensely colored than a solution prepared immediately before use by mixing 2.4 mL of ferric chloride CS and 0.6 mL of cobaltous chloride CS with
Diluent, prepared as directed below, to make 10.0 mL, and diluting 5.0 mL of the solution so obtained with
Diluent to make 10.0 mL. Make the comparison by viewing the substance and the solution downward in matched color-comparison tubes against a white surface (see
Color and Achromicity  631
631
).
Diluent—
Transfer 27.5 mL of hydrochloric acid to a 1000-mL volumetric flask, and dilute with water to volume.
Identification—
A:
It meets the requirements of the test for Saponification value.
B:
It meets the requirements of the test for Fatty acid composition.
Fatty acid composition  401
401 —
—
The fatty acid fraction of Medium-Chain Triglycerides exhibits the following composition, as determined in the section
Fatty Acid Composition. Disregard any peak with an area less than 0.05% of the total area:
| Carbon-Chain Length |
Number of Double Bonds |
Percentage (%) |
| 6 |
0 |
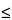 2.0 2.0 |
| 8 |
0 |
50.0–80.0 |
| 10 |
0 |
20.0–50.0 |
| 12 |
0 |
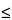 3.0 3.0 |
| 14 |
0 |
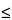 1.0 1.0 |
Viscosity  911
911 :
:
between 25 and 33 centipoises determined at 20 ± 0.1

with a capillary viscosimeter.
Alkaline impurities—
Dissolve 2.0 g of Medium-Chain Triglycerides in a mixture of 1.5 mL of alcohol and 3.0 mL of ethyl ether. Add 0.05 mL of
bromophenol blue TS, and titrate with 0.01 N hydrochloric acid to a yellow endpoint: not more than 0.15 mL of 0.01 N hydrochloric acid is required.
Heavy metals, Method II  231
231 —
[NOTE—
—
[NOTE—Use this test for Medium-Chain Triglycerides intended for use other than in parenteral nutrition.
]
Test solution—
Transfer 2.0 g of Medium-Chain Triglycerides to a quartz crucible, add 0.5 g of magnesium oxide, and mix. Ignite the crucible to dull redness until a homogeneous white or grayish-white mass is obtained. Ignite at 800

for 1 hour, cool, and dissolve the residue by adding two 5-mL portions of diluted hydrochloric acid. Add 0.1 mL of phenolphthalein TS and then ammonium hydroxide until a pink color is obtained. Cool, add glacial acetic acid until the solution is decolorized, then add 0.5 mL in excess, and dilute with water to 20.0 mL.
Standard solution—
To 0.5 g of magnesium oxide add 2.0 mL of
Standard Lead Solution, and evaporate to dryness at 105

for 1 hour. Using the same conditions as prescribed for the
Test solution, ignite, dissolve in diluted hydrochloric acid, add ammonia and then acetic acid, and dilute with water to 20.0 mL.
Procedure—
To 12 mL of the Test solution, add 2.0 mL of pH 3.5 Acetate Buffer, mix, add to 1.2 mL of thioacetamide-glycerin base TS, and mix immediately. To 10 mL of the Standard solution, add 2.0 mL of the Test solution, add 2.0 mL of pH 3.5 Acetate Buffer, mix, add to 1.2 mL of thioacetamide-glycerin base TS, and mix immediately. Prepare a blank, using a mixture of 10 mL of water and 2.0 mL of the Test solution. Compared to the blank, the Standard solution shows a light brown color. Dilute both the Test solution and the Standard solution with water to 50 mL, allow to stand for 2 minutes, and view downward over a white surface: any brown color from the Test solution is not darker than that of the solution from the Standard solution (not more than 10 µg per g).
Limit of chromium—
[NOTE—Use this test for Medium-Chain Triglycerides intended for use in parenteral nutrition.
]
Test stock solution—
Transfer about 50 g of Medium-Chain Triglycerides to a 100-mL volumetric flask, dissolve in and dilute with diisobutyl ketone to volume.
Test solution—
Transfer 4.0 mL of Test stock solution to a 10-mL volumetric flask, and dilute with diisobutyl ketone to volume.
Chromium standard solution—
Transfer about 0.283 g of potassium dichromate, previously dried at 105

for 4 hours and accurately weighed, to a 1000-mL volumetric flask, and dilute with water to volume. Immediately before use, dilute this solution with water to 1000 times its volume. This solution contains the equivalent of 0.1 µg of chromium per mL.
Standard solutions—
Into each of three 10-mL volumetric flasks, transfer 4.0 mL of Test stock solution, add 0.5, 1.0, and 2.0 mL, respectively, of Chromium standard solution, and dilute with diisobutyl ketone to volume. These solutions contain 0.005 µg, 0.01 µg, and 0.02 µg of chromium per mL, respectively.
Procedure—
Concomitantly determine the absorbances of the
Standard solutions and the
Test solution at least three times each, at the wavelength of maximum absorbance at 357.8 nm, with a suitable atomic absorption spectrophotometer (see
Spectrophotometry and Light-Scattering  851
851
) equipped with a graphite furnace and a chromium hollow-cathode lamp, using argon as the carrier gas. Record the average of the steady readings for each of the
Standard solutions and the
Test solution. Plot the absorbances of the
Standard solutions and the
Test solution versus the concentration of added chromium. Draw the straight line best fitting the points, and extrapolate the line until it meets the concentration axis. The distance between this point and the intersection of the axes represents the concentration of chromium in the
Test solution. Not more than 0.05 µg per g is found.
Limit of copper—
[NOTE—Use this test for Medium-Chain Triglycerides intended for use in parenteral nutrition.
]
Test stock solution and Test solution—
Proceed as directed in the test for Limit of chromium.
Copper standard solution—
Transfer about 0.393 g of cupric sulfate, accurately weighed, to a 1000-mL volumetric flask, and dilute with water to volume. Immediately before use, dilute this solution with water to 1000 times its volume. This solution contains the equivalent of 0.1 µg of copper per mL.
Standard solutions—
Into each of three 10-mL volumetric flasks, transfer 4.0 mL of Test stock solution, add 1.0, 2.0, and 4.0 mL, respectively, of Copper standard solution, and dilute with diisobutyl ketone to volume. These solutions contain 0.01 µg, 0.02 µg, and 0.04 µg of copper per mL, respectively.
Procedure—
Concomitantly determine the absorbances of the
Standard solutions and the
Test solution at least three times each, at the wavelength of maximum absorbance at 324.7 nm, with a suitable atomic absorption spectrophotometer (see
Spectrophotometry and Light-Scattering  851
851
) equipped with a graphite furnace and a copper hollow-cathode lamp, using argon as the carrier gas. Record the average of the steady readings for each of the
Standard solutions and the
Test solution. Plot the absorbances of the
Standard solutions and the
Test solution versus the concentration of added copper. Draw the straight line best fitting the points, and extrapolate the line until it meets the concentration axis. The distance between this point and the intersection of the axes represents the concentration of copper in the
Test solution. Not more than 0.1 µg per g is found.
Limit of lead—
[NOTE—Use this test for Medium-Chain Triglycerides intended for use in parenteral nutrition.
]
Test stock solution and Test solution—
Proceed as directed in the test for Limit of chromium.
Lead standard solution—
Dissolve 160 mg of lead nitrate in 100 mL of water that contains 1 mL of lead-free nitric acid, and dilute with water to 1000 mL. Pipet 10 mL of this solution into a 100-mL volumetric flask, dilute with water to volume, and mix. Immediately before use, dilute this solution with water to 100 times its volume. This solution contains the equivalent of 0.1 µg of lead per mL.
Standard solutions—
Into each of three 10-mL volumetric flasks, transfer 4.0 mL of Test stock solution, add 1.0, 2.0, and 4.0 mL, respectively, of Lead standard solution, and dilute with diisobutyl ketone to volume. These solutions contain 0.01 µg, 0.02 µg, and 0.04 µg of lead per mL, respectively.
Procedure—
Concomitantly determine the absorbances of the
Standard solutions and the
Test solution at least three times each, at the wavelength of maximum absorbance at 283.3 nm, with a suitable atomic absorption spectrophotometer (see
Spectrophotometry and Light-Scattering  851
851
) equipped with a graphite furnace coated inside with palladium carbide and a lead hollow-cathode lamp, using argon as the carrier gas. Calcination is carried out in the presence of oxygen at a temperature below 800

. Record the average of the steady readings for each of the
Standard solutions and the
Test solution. Plot the absorbances of the
Standard solutions and the
Test solution versus the concentration of added lead. Draw the straight line best fitting the points, and extrapolate the line until it meets the concentration axis. The distance between this point and the intersection of the axes represents the concentration of lead in the
Test solution. Not more than 0.1 µg per g is found.
Limit of nickel—
[NOTE—Use this test for Medium-Chain Triglycerides intended for use in parenteral nutrition.
]
Test stock solution and Test solution—
Proceed as directed in the test for Limit of chromium.
Nickel standard solution—
Immediately before use, dilute 10 mL of nickel standard solution TS with water to 1000 mL. This solution contains the equivalent of 0.1 µg of nickel per g.
Standard solutions—
Into each of three 10-mL volumetric flasks, transfer 4.0 mL of Test stock solution, add 1.0, 2.0, and 4.0 mL, respectively, of Nickel standard solution, and dilute with diisobutyl ketone to volume. These solutions contain 0.01 µg, 0.02 µg, and 0.04 µg of nickel per mL, respectively.
Procedure—
Concomitantly determine the absorbances of the
Standard solutions and the
Test solution at least three times each, at the wavelength of maximum absorbance at 232 nm, with a suitable atomic absorption spectrophotometer (see
Spectrophotometry and Light-Scattering  851
851
) equipped with a graphite furnace and a nickel hollow-cathode lamp, using argon as the carrier gas. Record the average of the steady readings for each of the
Standard solutions and the
Test solution. Plot the absorbances of the
Standard solutions and the
Test solution versus the concentration of added nickel. Draw the straight line best fitting the points, and extrapolate the line until it meets the concentration axis. The distance between this point and the intersection of the axes represents the concentration of nickel in the
Test solution. Not more than 0.1 µg per g is found.
Limit of tin—
[NOTE—Use this test for Medium-Chain Triglycerides intended for use in parenteral nutrition.
]
Test stock solution and Test solution—
Proceed as directed in the test for Limit of chromium.
Tin standard solution—
Dissolve 500 mg of metallic tin (Sn), accurately weighed, in a mixture of 5 mL of water and 25 mL of hydrochloric acid, and dilute with water to 1000 mL. Immediately before use, dilute 10 mL of this solution with dilute hydrochloric acid (2.5 in 100) to 1000 mL, and then dilute 10 mL of the solution so obtained with water to 500 mL. This solution contains the equivalent of 0.1 µg of tin per g.
Standard solutions—
Into each of three 10-mL volumetric flasks, transfer 4.0 mL of Test stock solution, add 1.0, 2.0, and 4.0 mL, respectively, of Tin standard solution, and dilute with diisobutyl ketone to volume. These solutions contain 0.01 µg, 0.02 µg, and 0.04 µg of tin per mL, respectively.
Procedure—
Concomitantly determine the absorbances of the
Standard solutions and the
Test solution at least three times each, at the wavelength of maximum absorbance at 286.3 nm, with a suitable atomic absorption spectrophotometer (see
Spectrophotometry and Light-Scattering  851
851
) equipped with a graphite furnace coated inside with palladium carbide and a tin hollow-cathode lamp, using argon as the carrier gas. Record the average of the steady readings for each of the
Standard solutions and the
Test solution. Plot the absorbances of the
Standard solutions and the
Test solution versus the concentration of added tin. Draw the straight line best fitting the points, and extrapolate the line until it meets the concentration axis. The distance between this point and the intersection of the axes represents the concentration of tin in the
Test solution. Not more than 0.1 µg per g is found.