Assay for lactase activity—
pH 4.5 Acetate buffer—
Dilute 57.5 mL of glacial acetic acid with sufficient water to make 500 mL of solution. Transfer 50 mL of the glacial acetic acid solution into a 1000-mL volumetric flask, add 11.3 mL of 4 N sodium hydroxide, and dilute with water to volume. If necessary, adjust with the addition of either the glacial acetic acid solution or 4 N sodium hydroxide to a pH of 4.50 ± 0.05.
Substrate solution—
On the day of use, weigh 370.0 mg of
o-nitrophenyl-
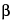
-
D-galactopyranoside, and place in a 100-mL volumetric flask. Add about 50 mL of
pH 4.5 Acetate buffer, swirl to dissolve, then dilute with
pH 4.5 Acetate buffer to volume.
Standard preparation—
Transfer about 0.4 g of
USP Lactase RS, accurately weighed, to a 1000-mL volumetric flask. Add about 600 mL of water, allow to stand for 15 minutes, swirl gently, and dilute with water to volume. Pipet 3.0 mL of this solution into a 200-mL volumetric flask, and dilute with water to volume.
Assay preparation—
Transfer an accurately weighed quantity of about 0.4 g of Lactase to a 1000-mL volumetric flask. Add about 600 mL of water, allow to stand for 15 minutes, swirl gently, and dilute with water to volume. Pipet 3.0 mL of this solution into a 200-mL volumetric flask, and dilute with water to volume.
Procedure—
Transfer 2.0 mL of the
Substrate solution to 3 separate test tubes labeled S, U, and B. Transfer the tubes to a thermostated water bath maintained at 37.0 ± 0.1

, and incubate for 10 minutes. Following the incubation, add rapidly to tube S, 0.5 mL of the
Standard preparation, to tube U, 0.5 mL of the
Assay preparation, and to tube B (the reagent blank), 0.5 mL of water. Mix each tube on a vortex mixer for 1 second, and immediately return the tubes to the water bath, which has been maintained at 37.0 ± 0.1

. After 15 minutes of incubation, rapidly add 2.5 mL of a 10% sodium carbonate solution to each test tube to stop the enzyme reaction. Add 20.0 mL of water to each test tube, and mix. Concomitantly determine the absorbances of the three solutions at 420 nm in a 1-cm cell using a suitable spectrophotometer (see
Spectrophotometry  851
851
). Calculate the number of USP Lactase Units of the Lactase taken by the formula:
(
P)(
WS /
WU)(
AU  AB
AB) / (
AS  AB
AB),
in which
P is the potency of
USP Lactase RS in USP Units per g;
WS is the amount, in g, of
USP Lactase RS taken;
WU is the amount, in g, of the Lactase taken; and
AU is the absorbance reading of tube U,
AB is the absorbance reading of tube B, and
AS is the absorbance reading of tube S.