Related compounds—
Use the chromatograms of the
Assay preparation and
Standard preparation 2 as obtained in the
Assay.
Calculate the percentage of each related compound having the greatest response, other than erythromycin A, erythromycin B, erythromycin C, erythromycin A enol ether, and pseudoerythromycin A enol ether (retention time relative to erythromycin A peak is about 1.5), in the portion of Erythromycin Stearate taken by the formula:
30(CS2P/W)(ri / rS2),
in which
CS2 is the concentration, in mg per mL, of
USP Erythromycin RS in
Standard preparation 2; P is the designated percentage of erythromycin A in
USP Erythromycin RS;
W is the quantity, in mg, of Erythromycin Stearate taken to prepare the
Assay preparation; ri is the peak response of each related compound, other than erythromycin A, erythromycin B, erythromycin C, erythromycin A enol ether, and pseudoerythromycin A enol ether, observed in the chromatogram obtained from the
Assay preparation; and
rS2 is the erythromycin A peak response in the chromatogram obtained from
Standard preparation 2; not more than 3.0% of any individual related compound is found.
Calculate the percentage of erythromycin A enol ether in the portion of Erythromycin Stearate taken by the formula:
(30 / 11)(CS2P / W)(rE / rS2),
in which 11 is the response factor for erythromycin A enol ether in relation to that of erythromycin A; rE is the peak response of the erythromycin A enol ether observed in the chromatogram obtained from the Assay preparation; and the other terms are as defined above: not more than 3.0% of erythromycin A enol ether is found.
Calculate the percentage of pseudoerythromycin A enol ether in the portion of Erythromycin Stearate taken by the formula:
(30 / 6.6)(CS2P / W)(rP / rS2),
in which 6.6 is the response factor for pseudoerythromycin A enol ether in relation to that of erythromycin A; rP is the peak response of the pseudoerythromycin A enol ether (retention time relative to the erythromycin A peak is about 1.5) observed in the chromatogram obtained from the Assay preparation; and the other terms are as defined above: not more than 3.0% of pseudoerythromycin A enol ether is found.
Assay—
pH 8.0 Buffer—
Prepare a solution of dibasic potassium phosphate (2 in 100), and adjust with phosphoric acid to a pH of 8.0.
pH 9.0 Buffer—
Prepare a solution of dibasic potassium phosphate (3.5 in 100), and adjust with
potassium hydroxide TS or diluted phosphoric acid (1 in 10), as appropriate, to a pH of 9.0.
pH 3.5 Buffer—
Adjust 20 mL of
pH 8.0 Buffer with phosphoric acid to a pH of 3.5.
Mobile phase—
Mix 50 mL of
pH 9.0 Buffer with 400 mL of water, add 175 mL of tertiary butyl alcohol and 30 mL of acetonitrile, dilute with water to 1000 mL, and mix. Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
NOTE—Use the following solutions promptly, or within 1 day if stored in a refrigerator.
Standard preparation 1—
Transfer about 40 mg of
USP Erythromycin RS, accurately weighed, to a conical flask, add 5.0 mL of methanol, and swirl to dissolve. Add 5.0 mL of
pH 8.0 Buffer, and mix.
Erythromycin A enol ether solution—
Dissolve about 5 mg of
USP Erythromycin RS in 1 mL of methanol. Add 5 mL of
pH 3.5 Buffer, mix, and allow to stand for about 30 minutes.
Assay preparation—
Transfer about 165 mg of Erythromycin Stearate, accurately weighed, to a 100-mL conical flask, add 15.0 mL of methanol, and swirl to dissolve. Add 15.0 mL of
pH 8.0 Buffer, and mix. Allow the resulting suspension to settle, and pass a portion of the supernatant through a filter having a 0.2-µm or finer porosity. Use the clear filtrate.
Chromatographic system
(see
Chromatography  621
621
)—The liquid chromatograph is equipped with a 215-nm detector and a 4.6-mm × 25-cm column that contains packing L21 (1000
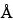
) and is maintained at a constant temperature of about 70

. The flow rate is about 2 mL per minute. Chromatograph
Standard preparation 2, and record the peak responses as directed for
Procedure: the order of elution of the components is erythromycin related compound N, erythromycin C, erythromycin A, and erythromycin B; and the resolution,
R, between erythromycin related compound N and erythromycin C is not less than 0.8 and between erythromycin related compound N and erythromycin A not less than 5.5. Chromatograph the
Erythromycin A enol ether solution, and adjust the duration of chromatography to include the erythromycin A enol ether peak, which has a retention time of about 4.3 to 4.7 times that of erythromycin A. Chromatograph
Standard preparation 1, and record the peak responses as directed for
Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 100 µL) of
Standard preparation 1, Standard preparation 2, and the
Assay preparation into the chromatograph; record the chromatograms for a period of time that is adequate to include the erythromycin A enol ether peak, if present; and measure the areas of the peak responses. Calculate the percentage of erythromycin A in the portion of Erythromycin Stearate taken by the formula:
30(CS1P / W)(rU / rS1),
in which
CS1 is the concentration, in mg per mL, of
USP Erythromycin RS in
Standard preparation 1; P is the designated percentage of erythromycin A in
USP Erythromycin RS;
W is the quantity, in mg, of Erythromycin Stearate taken to prepare the
Assay preparation; and
rU and
rS1 are the erythromycin A peak responses in the chromatograms obtained from the
Assay preparation and
Standard preparation 1, respectively.
Calculate the percentage of erythromycin B and erythromycin C in the portion of Erythromycin Stearate taken by the formula:
30(CS2P / W)(rU / rS2),
in which CS is the concentration, in mg per mL, of the relevant USP Reference Standard in Standard preparation 2; P is the designated percentage of erythromycin B or erythromycin C in the relevant USP Reference Standard; W is the quantity, in mg, of Erythromycin Stearate taken to prepare the Assay preparation; and rU and rS2 are the peak responses of the relevant analyte in the chromatograms obtained from the Assay preparation and Standard preparation 2, respectively. The percentage of erythromycin B is not more than 12.0%; and the percentage of erythromycin C is not more than 5.0%.