Identification—
A:
The retention times of the major peaks in the chromatogram of the
Assay preparation correspond to those in the chromatogram of the
Standard preparation, both relative to the internal standard as obtained in the
Assay.
B:
Using a sharp blade, carefully open 1 Capsule and transfer the entire contents into a 250-mL flask. Inspect the encapsulated liquid to ensure that crystallization or agglomeration of the drug substance has not taken place. Add 5 mL of water to the flask, and swirl to dissolve. Add 10 mL of p-dimethylaminobenzaldehyde TS: a blue color develops within 2 minutes and persists for not less than 10 minutes.
Microbial limits  61
61 —
—
The total bacterial count does not exceed 1000 per g, and the total combined molds and yeasts count does not exceed 200 per g. Capsules meet also the requirements of the tests for absence of
Salmonella species,
Escherichia coli,
Staphylococcus aureus, and
Pseudomonas aeruginosa. The test specimen shows an absence of the members of the
Enterobacteriaceae family and
Pseudomonadaceae family at levels greater than 100 per g of each.
Dissolution  711
711 —
—
Medium:
water; 500 mL.
Apparatus 2:
50 rpm.
Time:
15 minutes.
Procedure—
Place 1 Capsule in each vessel, and allow the Capsule to sink to the bottom of the vessel before starting rotation of the blade. Observe the Capsules, and note if there is membrane formation. Record the time that each Capsule ruptures.
Tolerances—
All of the Capsules tested rupture in not more than 15 minutes. If 1 or 2 Capsules fail to rupture in 15 minutes but rupture in not more than 30 minutes, repeat the test on 12 additional Capsules. Not more than 2 of the total of 18 Capsules tested rupture in more than 15 but not more than 30 minutes.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of water, acetonitrile, and triethylamine (32:18:1). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Internal standard solution—
Dissolve an accurately weighed quantity of m-chloroacetanilide in acetonitrile to obtain a solution having a known concentration of about 0.12 mg per mL.
Tartaric acid solution—
Dissolve an accurately weighed quantity of tartaric acid in water to obtain a solution having a known concentration of about 5.6 mg per mL.
Standard preparation—
Transfer about 25.0 mg of
USP Ergoloid Mesylates RS, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with
Internal standard solution to volume, and mix. Transfer 40.0 mL of this solution to a 200-mL volumetric flask, add 40.0 mL of
Tartaric acid solution, and mix.
Assay preparation—
Pipet 40.0 mL of
Tartaric acid solution into a 200-mL volumetric flask, and heat in a water bath maintained at 50

. Add 10 Capsules (or the equivalent of 10 mg of ergoloid mesylates), and shake the flask by mechanical means for 10 minutes or until the gelatin has dissolved. Pipet 40.0 mL of
Internal standard solution into the flask, and shake for an additional 10 minutes. Remove the flask from the bath and cool to room temperature. Transfer about 20 mL of the solution to a 30-mL centrifuge tube, and centrifuge at 10,000 rpm for 60 minutes. Filter a portion of the supernatant through a filter having a porosity of 0.45 µm, discarding the first 5 mL of the filtrate. Use the remainder of the filtrate as the
Assay preparation.
Chromatographic system
(see
Chromatography  621
621
)—The liquid chromatograph is equipped with a 280-nm detector and an 8-mm × 10-cm column that contains packing L1. The flow rate is about 2 mL per minute. Chromatograph the
Standard preparation, and record the peak responses as directed under
Procedure: the relative retention times for
m-chloroacetanilide, dihydroergocornine, dihydro-

-ergocryptine, dihydroergocristine, and dihydro-
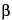
-ergocryptine are about 1.0, 1.4, 1.8, 2.2, and 2.8, respectively; the column efficiency determined for the dihydro-
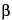
-ergocryptine peak is not less than 950 theoretical plates; the tailing factor for dihydro-
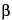
-ergocryptine is not more than 2.5 and that for dihydroergocornine is not more than 2.0; the resolution,
R, between dihydro-

-ergocryptine and dihydroergocristine is not less than 1.35; and the relative standard deviation of the sum of the four ergoloid mesylate peaks for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 50 µL) of the
Standard preparation and the
Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the total quantity, in mg, of ergoloid mesylates in the portion of Capsules taken by the formula:
80C(SRU / SRS),
in which
C is the concentration, in mg per mL, of
USP Ergoloid Mesylates RS in the
Standard preparation, and
SRU and
SRS are sums of the ratios of the peak responses of the individual alkaloids to the peak response of the internal standard obtained from the
Assay preparation and the
Standard preparation, respectively. Calculate the percentage of each of the individual alkaloids in the portion of Capsules taken by the formula:
100RU(MW)U / S[RU(MW)U],
in which (
MW)
U is the molecular weight of the individual alkaloid.