2-Naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, monohydrochloride [4
S-(4

,4a

,5a

,6
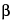
,12a

)]-.
7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride



[
64-72-2].