C
17H
21NO
4·HBr·3H
2O





438.31
Benzeneacetic acid,

-(hydroxymethyl)-, 9-methyl-3-oxa-9-azatricyclo[3.31.0.
2,4]non-[7-yl ester, hydrobromide, trihydrate, [7(
S)-(1

,2
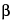
,4
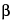
,5

,7
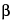
)]-.
6
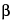
,7
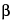
-Epoxy-1
 H
H,5
 H
H-tropan-3

-ol (

)-tropate (ester) hydrobromide trihydrate



[
6533-68-2].
Anhydrous
384.27



[
114-49-8].
Packaging and storage—
Preserve in tight, light-resistant containers.
Change to read:
Identification—
B:
To 1 mL of a solution (1 in 20) add a few drops of chlorine TS, and shake the mixture with 1 mL of chloroform: the latter assumes a brownish color.
Specific rotation  781S
781S :
:
between

24

and

26

.
Test solution:
an amount equivalent to 50 mg of anhydrous Scopolamine Hydrobromide per mL, in water.
pH  791
791 :
:
between 4.0 and 5.5, in a solution (1 in 20).
Limit of apoatropine—
To 15 mL of a solution (1 in 100) add 0.05 mL of 0.1 N potassium permanganate VS: the solution is not completely decolorized within 5 minutes.
Other foreign alkaloids—
To 1 mL of a solution (1 in 20) add a few drops of 6 N ammonium hydroxide: no turbidity is produced. Add 1 N potassium hydroxide to another 1-mL portion of the solution: only a transient whitish turbidity is produced.
Organic volatile impurities, Method I  467
467 :
:
meets the requirements.
Assay—
Dissolve about 750 mg of Scopolamine Hydrobromide, accurately weighed, in a mixture of 30 mL of glacial acetic acid and 10 mL of
mercuric acetate TS, warming slightly to effect solution. Cool the solution to room temperature, add 2 drops of
crystal violet TS, and titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 38.43 mg of C
17H
21NO
4·HBr.
Auxiliary Information—
Staff Liaison :
Feiwen Mao, M.S., Senior Scientific Associate
Expert Committee : (MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals
USP29–NF24 Page 1949
Pharmacopeial Forum : Volume No. 31(1) Page 73
Phone Number : 1-301-816-8320