Peptide mapping—
Mobile phase—
Prepare separate filtered and degassed solutions consisting of a solution (1 in 1000) of trifluoroacetic acid in water
(Solution A) and a 1 in 1000 solution of trifluoroacetic acid in acetonitrile
(Solution B). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Digestion solution—
Dissolve 29.4 mg of calcium chloride and 1.8 mg of
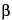
-alanine in 2 mL of water. Adjust with hydrochloric acid to a pH of 4.0. Add 0.4 mg of trypsin, and mix.
Standard solution—
Dissolve an accurately weighed quantity of USP Sargramostim RS in water, and dilute quantitatively with water to obtain a solution having a known concentration of about 500 µg per mL. Transfer 100 µL of this solution to a clean test tube, and add 11 µL of pH 7.6 buffer solution (see
Buffer Solutions in the section
Reagents, Indicators, and Solutions) containing 0.1 M tris(hydroxymethyl)aminomethane. Add 25 µL of
Digestion solution, and incubate at 37

for 2 hours. Quench the reaction by adding 3 µL of 20% trifluoroacetic acid.
Test solution—
Using an accurately weighed quantity of Sargramostim, proceed as directed for Standard solution.
Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a 220-nm detector and a 25-cm × 4.6-mm column that contains 10-µm packing L1. The column is maintained at ambient temperature, and the flow rate is about 1 mL per minute. The system is programmed to provide variable mixtures of
Mobile phase. Equilibrate the system with a
Mobile phase consisting of 100%
Solution A. After injection of the solution under test, the composition is changed linearly at a rate of 1% per minute over the next 35 minutes so that it then consists of 65%
Solution A and 35%
Solution B, and then changed linearly at a rate of 2% per minute over the next 15 minutes to a mixture of 35%
Solution A and 65%
Solution B.
Procedure—
Separately inject equal volumes (about 100 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the eight major peaks as defined in the USP Sargramostim RS Data Sheet. The retention times of the peak responses from the Test solution correspond to those from the Standard solution if the retention times of corresponding peaks do not differ by more than 0.3 minutes, the peak areas ratios for peaks 4, 8, and 10 are between 0.7 and 1.3, and no additional significant peaks or shoulders are found.
Chromatographic purity—
Mobile phase—
Prepare separate filtered and degassed solutions consisting of a solution (1 in 1000) of trifluoroacetic acid in water
(Solution A), a 1 in 1000 solution of trifluoroacetic acid in acetonitrile
(Solution B), and a solution prepared by dissolving 116.9 g of sodium chloride in 2000 mL of water and adding 2 mL of trifluoroacetic acid
(Solution C). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Standard solution—
Dissolve an accurately weighed quantity of USP Sargramostim RS in water, and dilute quantitatively with water to obtain a solution having a known concentration of about 1.0 mg per mL.
Test solution—
Using an accurately weighed quantity of Sargramostim, prepare as directed for Standard solution.
Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 25-cm column that contains packing L1. The flow rate is about 1 mL per minute. The system is programmed to provide a
Mobile phase consisting of a mixture containing variable proportions of
Solution A and
Solution B, with a constant 20% of
Solution C. Equilibrate the system with a mixture of 55%
Solution A, 25%
Solution B, and 20%
Solution C. After injection of the
Test solution, the proportion of
Solution A is decreased linearly from 55% to 15% and the proportion of
Solution B is increased linearly from 25% to 65% at a rate of 1% per minute.
Procedure—
Separately inject equal volumes (about 50 µL) of the
Standard solution and the
Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. The major peaks are from hyperglycosylated sargramostim and from the three glycosylated forms of Sargramostim, as indicated in the USP Sargramostim RS Data Sheet. The peak responses from the
Test solution correspond to those from the
Standard solution, and no peaks or shoulders are present in the chromatogram of the
Test solution that are not present in the chromatogram of the
Standard solution. Calculate the percentage of hyperglycosylated sargramostim in the
Test solution by the formula:
100(rU / rs),
in which
rU is the peak response for hyperglycosylated sargramostim, and
rs is the sum of the responses of all of the sargramostim peaks: not more than 5.6% is found. Calculate the percentage of each of the three glycosylated forms of Sargramostim in the
Test solution by the formula:
100(ri / rs),
in which
ri is the peak response for each individual glycosylated form of Sargramostim, and
rs is the sum of the peak responses of all three glycosylated forms of Sargramostim. The percentages of each of the three glycosylated forms of Sargramostim, in order of elution, are between 25% and 42%, between 14% and 32%, and between 35% and 53%.
Protein content—
Standard solutions—
Dissolve accurately weighed quantities of USP Sargramostim RS in water to obtain solutions having known concentrations of about 100, 200, 400, 600, 800, and 1000 µg per mL.
Test solution—
Dissolve an accurately weighed quantity of Sargramostim in water to obtain a solution having a concentration of between 250 and 500 µg per mL.
BCA reagent—
Dissolve 10 g of bicinchoninic acid, 20 g of sodium carbonate monohydrate, 1.6 g of sodium tartrate, 4 g of sodium hydroxide, and 9.5 g of sodium bicarbonate in water. Adjust, if necessary, with sodium hydroxide or sodium bicarbonate to a pH of 11.25. Dilute with water to 1 L, and mix.
Copper sulfate reagent—
Dissolve 2 g of cupric sulfate in water, and dilute with water to 50 mL.
Copper-BCA reagent—
Mix 1 mL of Copper sulfate reagent and 50 mL of BCA reagent. [NOTE—If a commercially available kit is used, follow the manufacturer's instructions for preparation of the Copper-BCA reagent.]
Procedure—
Mix 0.1 mL of each
Standard solution, the
Test solution, and 0.1 mL of water to provide the blank with 2 mL of the
Copper-BCA reagent. Incubate the solutions at 37

for 30 minutes, and allow to stand for 5 minutes at room temperature. Within 60 minutes following incubation, determine the absorbances of the
Standard solutions and the
Test solution in 1-cm cells at 562 nm, with a suitable spectrophotometer (see
Spectrophotometry and Light-scattering  851
851
), using the blank to set the instrument to zero. Plot the absorbances of the
Standard solutions versus the concentrations, in µg per mL, of USP Sargramostim RS, and draw the straight line best fitting the plotted points. From the graph so obtained, determine the concentration, in µg per mL, of protein in the
Test solution.
Assay—
Iscove's Modified Dulbecco's Medium—
Prepare a mixture of the ingredients in the quantities shown in sufficient water to obtain 1 L of medium, and sterilize by filtration.
Prepare aseptically, sterilize by filtration, and store at 2

to 8

. Use within one month.
Medium B—
Dissolve an accurately weighed quantity of USP Sargramostim RS in
Medium A, and dilute quantitatively with
Medium A to obtain a solution having a known concentration of about 1 µg per mL. Prepare aseptically, sterilize by filtration, and store at 2

to 8

. Use within one month.
Standard preparation—
Dissolve an accurately weighed quantity of USP Sargramostim RS in
Medium A, and dilute quantitatively with
Medium A to obtain a solution having a known concentration of about 100 ng per mL. Dispense aseptically in equal portions, accurately measured, and store at

60

or below. Use within 24 months. Store thawed portions at a temperature between 2

and 8

, and use within one month. At the time of use, dilute with
Medium A to obtain a solution having a known concentration of about 2 ng per mL.
Assay preparation—
Dissolve an accurately weighed quantity of Sargramostim in Medium A, and dilute quantitatively with Medium A to obtain a solution containing about 2 ng per mL.
Cell culture preparation—
Prepare cell cultures of [TF-1 cells (ATCC CRL-2003)]. Passage the cultures every 3 to 4 days, using a 1:10 subculture of the cells for up to 3 months. After 3 months, initiate a new culture. Use Medium A containing 0.5% Medium B for passage propagation and storage in the frozen state.
Cell suspension—
Wash the cells three times in Medium A, and adjust the cell concentration to 5 × 104 cells per mL in Medium A.
Tritiated thymidine solution—
Use a tritiated thymidine stock solution having a concentration of 20 Ci per mmol. Add 1.0 mL of the tritiated thymidine stock solution to 49 mL of
Medium A, and store at 2

to 8

. Use within 2 weeks.
Procedure—
Use a 96-well, flat-bottom microtitration plate with wells arranged in 8 rows (labeled A through H) with 12 wells (numbered 1 to 12) in each row. Place 50 µL of
Medium A in wells 2 to 12. Place 100 µL of the
Standard preparation, or each
Assay preparation or
Medium A (negative control) in well 1. Make serial dilutions by transferring 50 µL from well 1 to well 2, and so on through well 12 (serial twofold dilutions). Place 50 µL of the
Cell suspension in each well, and incubate the microtitration plate for 72 hours at 37

in a 10% carbon dioxide incubator. Following incubation, add 25 µL of
Tritiated thymidine solution to each well, and return the plate to the same incubator for an additional 4 to 5 hours. Before harvesting the cells on a filter mat, prewet the mat filter, using distilled water.
[NOTE—The prewetting minimizes background radiation noise.
] Using a multiple automated sample harvesting system, place the incubated plate under the harvesting system. Fill the wells to the top with deionized water. Aspirate the water, and pass it through the collecting filter mat. Repeat the procedure at least 5 times, or until all the cells have been fully harvested. When all wells have been fully harvested, pour 5 to 10 mL of alcohol on the plate tray, and aspirate the methanol. Repeat the procedure if further drying of the filter mat is desired.
[NOTE—The alcohol helps to dry out the filter mat by carrying away the wash fluid.
] Remove the filter mat, and repeat the procedure until all plates under test have been harvested.
Dry the filter mat in a drying oven for about 30 minutes. Place the completely dry filter mats in a beta counter, and determine the amount of radioactivity in each cell well.
Convert the amount of incorporated radioactivity in each well to a percentage of the maximum incorporated radioactivity. If fewer than 5 values are between 3% and 97% of the maximum revision, repeat the Assay. Using the least squares method of regression analysis, plot the slope of each test specimen versus the slope of the standard, excluding any values exceeding the maximum of each dilution set. Calculate the Sargramostim Units in each mL of the Assay preparation in terms of the dilution that gives half-maximal activity. To convert this value to units of protein per mg, divide the Sargramostim Units per mL by the weight, in mg per mL, of protein in the initial undiluted solution.