Test solution—
Transfer an accurately weighed quantity of Capsules, equivalent to about 100 mg of the labeled amount of Extract, into a 100-mL, round-bottomed flask. Add 2.0 mL of
Internal standard solution and 20 mL of diluted hydrochloric acid. Attach a condenser, and reflux in a bath at 100

for 30 minutes. Cool the solution to room temperature, and adjust by the addition of about 5 mL of 10 N sodium hydroxide to a pH of 8. Extract twice using 50 mL of ether each time, wash the collected organic phases with 50 mL of water, and evaporate the organic phase to dryness under vacuum. Dissolve the residue with 4 mL of chloroform, and transfer to a cartridge containing 500 mg of packing L8 that has been conditioned with a 2-column volume of
n-hexane.
[NOTE—A suitable cartridge is Chromabond NH2, manufactured by Macheray Nagel, or equivalent.
] Collect the eluate. Elute twice with a 1-column volume of a mixture of chloroform and isopropanol (2:1). Combine the eluates, and evaporate to dryness. Dissolve the residue in 10 mL of chloroform. Evaporate about 500 µL of this solution to dryness under a stream of nitrogen. Dissolve the residue with 80 µL of
Derivatizing solution and 20 µL of pyridine. Allow to stand for not less than 10 minutes at room temperature.
Procedure—
Proceed as directed in
Pygeum Extract. Separately calculate the content, in mg, of campesterol, stigmasterol, and
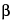
-sitosterol, respectively, in the portion of Capsules taken by the formula:
2C(RU / RS),
in which
C is the concentration of
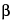
-sitosterol, in mg per mL, in the
Standard solution; RU is the ratio of the appropriate sterol peak to the internal standard in the chromatogram of the
Test solution; and
RS is the ratio of the
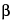
-sitosterol peak to the 5

-cholestane internal standard in the chromatogram of the
Standard solution. Calculate the total content of sterols, in mg, by adding the individual contents.