Ergotaman-3
¢,6
¢,18-trione, 9,10-dihydro-12
¢-hydroxy-2
¢,5
¢-bis(1-methylethyl)-, (5
¢
,10

)-, monomethanesulfonate (salt) mixture with 9,10

-dihydro-12
¢-hydroxy-2
¢-(1-methylethyl)-5
¢
-(phenylmethyl)ergotaman-3
¢,6
¢,18-trione monomethanesulfonate (salt), 9,10

-dihydro-12
¢-hydroxy-2
¢-(1-methylethyl)-5
¢
-(2-methylpropyl)ergotaman-3
¢,6
¢,18-trione monomethanesulfonate (salt), and 9,10

-dihydro-12
¢-hydroxy-2
¢-(1-methylethyl)-5
¢
-(1-methylpropyl)ergotaman-3
¢,6
¢,18-trione monomethanesulfonate (salt).
Dihydroergotoxine monomethanesulfonate (salt).
An equiproportional mixture of dihydroergocornine mesylate, dihydroergocristine mesylate, and ratio of dihydro-

-ergocryptine mesylate to dihydro-
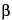
-ergocryptine mesylate is (1.5-2.5:1)