(C
19H
18ClN
3O
5S)
2·C
16H
20N
2





1112.11
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[[3-(2-chlorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-, [2
S-(2

,5

,6
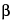
)]-, compd. with
N,
N¢-bis(phenyl-methyl)-1,2-ethanediamine (2:1).
(2
S,5
R,6
R)-6-[3-(
o-Chlorophenyl)-5-methyl-4-isoxazolecarboxamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with
N,
N¢-dibenzylethylenediamine (2:1)



[
23736-58-5].
Packaging and storage—
Preserve in tight containers.
Labeling—
Label it to indicate that it is for veterinary use only. Where it is intended for use in preparing sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of sterile dosage forms.
Identification—
Solutions:
Obtain the test solution as follows. To about 20 mg in a 50-mL conical flask, add 5 mL of 5 N sodium hydroxide, and heat on a steam bath for 20 minutes. Cool, transfer 1 mL of this solution to a separator containing 10 mL of 1.2 N sulfuric acid, and extract with 50 mL of ether. Wash the ether extract with 30 mL of water, and extract the ether layer with 50 mL of 0.1 N sodium hydroxide. Similarly prepare the Standard solution from about 15 mg of
USP Cloxacillin Sodium RS.
pH  791
791 :
:
between 3.0 and 6.5, in a suspension containing 10 mg per mL.
Sterility  71
71 —
—
Where the label states that Cloxacillin Benzathine is sterile, it meets the requirements when tested as directed for
Direct Inoculation of the Culture Medium under
Test for Sterility of the Product to be Examined, except to use Fluid Thioglycollate Medium containing polysorbate 80 solution (1 in 200) and an amount of sterile penicillinase sufficient to inactivate the cloxacillin in each tube, to use Soybean–Casein Digest Medium containing polysorbate 80 solution (1 in 200) and an amount of sterile penicillinase sufficient to inactivate the cloxacillin in each tube, and to shake the tubes once daily.
Assay—
Proceed as directed for cloxacillin under
Antibiotics—Microbial Assays  81
81
, using an accurately weighed quantity of Cloxacillin Benzathine dissolved quantitatively in methanol to yield a stock solution having a convenient concentration. Promptly dilute an accurately measured volume of this stock solution quantitatively and stepwise with
Buffer No. 1 to obtain a
Test Dilution having a concentration assumed to be equal to the median dose level of the Standard.
Auxiliary Information—
Staff Liaison :
Ian DeVeau, Ph.D., Associate Director
Expert Committee : (VET05) Veterinary Drugs 05
USP29–NF24 Page 571
Pharmacopeial Forum : Volume No. 31(4) Page 1050
Phone Number : 1-301-816-8178