1
Further information about guidances can be obtained by contacting the United States Food and Drug Administration, Division of Bioequivalence, Office of Generic Drugs, 7500 Standish Place, Metro Park North, Rockville, MD 20855 [Phone: (301) 594-2290; FAX: (301) 594-0181]. Copies of the guidances can be obtained from the United States Food and Drug Administration, Center for Drug Evaluation and Research, Consumer Affairs Branch HFD-210 5600 Fishers Lane, Rockville, MD 20857 [Phone: (301) 827-4573, FAX: (301) 827-4577.]
2
This statement, prepared by the Division of Bioequivalence, Office of Generic Drugs (OGD), in consultation with the Division of Biometrics, Office of Epidemiology and Biostatistics, is an informal communication under 21 CFR 10.90 (b)(9) that represents the best judgment of the Division of Bioequivalence and the Office at this time. This statement does not necessarily represent the formal position of the Center for Drug Evaluation and Research (CDER), the Food and Drug Administration (FDA), and does not bind or obligate CDER or FDA to the views expressed.
3
Note that a more general equation can be written for any multi-compartmental model as AUC
0–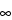
=
FD / (V
d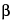 LZ
LZ), where
Vd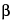
is the volume of distribution relating drug concentration in plasma or blood to the amount of drug in the body during the terminal exponential phase, and
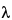 Z
Z is the terminal slope of the concentration-time curve.
9
Lund RE. Tables for an approximate test for outliers in linear models. Technometrics 1975; 17:473-476.
4
This statement, prepared by the FDA Division of Bioequivalence in the Office of Generic Drugs, is an informal communication under 21 CFR 10.90(b)(9) that represents the best judgment of the Division at this time. This statement does not necessarily represent the formal position of the Center for Drug Evaluation and Research, Food and Drug Administration, and does not bind or otherwise obligate the Center for Drug Evaluation and Research, Food and Drug Administration, to the views expressed.
6
Each subject should consume a standardized, high-fat breakfast consisting of the following:
1 buttered English muffin
1 slice of American cheese
1 slice of Canadian bacon
1 serving of hash brown potatoes
8 fluid oz. (240 mL) of whole milk
6 fluid oz. (180 mL) of orange juice
5
The sponsoring firm is advised that an Investigational New Drug (IND) application may be required if dosing levels exceed those recommended in the official labeling. See Policy and Procedure Guide 36-92, “Submission of an `Investigational New Drug Application' to the FDA Office of Generic Drugs (OGD)” and 21 CFR 312.2 and 320.31(b)(1).
7
Glipizide (5 and 10 mg) and glyburide (1.25, 2.5, and 5 mg), the second generation sulfonylurea antidiabetic agents, are comparatively more potent than tolbutamide (250 and 500 mg) and tolazamide (100, 250, and 500 mg). Therefore, in a fasting bioequivalence study involving normal subjects, hypoglycemic events occur more frequently with glipizide and glyburide than with tolbutamide and tolazamide. In the case of glipizide, the hypoglycemic episodes in normal subjects participating in a fasting bioequivalence study were fewer when the glucose was given to subjects every 15 minutes than when it was given every 30 minutes. In a study with such a design, measurement of plasma glucose is not necessary because it will not reflect the pharmacodynamic endpoint. However, such design is preferable to the usual fasting study design to ensure the welfare of the subjects and to avoid excessive drop out rate.
8
Each subject should consume the following high-fat breakfast:
2 slices of toasted white bread spread with butter
2 oz. of hash brown potatoes
8 fluid oz. (240 mL) of whole milk